Last Updated: August 2023
PROJECT DESCRIPTION
BACKGROUNDSupported
catalysts
are
essential
components
in
a
variety
of
industrial
processes,
ranging
from
catalytic
converters
to
production
of
new
drugs.
The
performance
of
a
catalytic
process
is
intimately
related
to
the
catalyst
design
-
uniform,
egg-
yolk,
egg
-
shell
and
egg
-
white
metal
profiles.
It
is
generally
believed
that
the
metal
profile
is
controlled
by
the
conditions
that
are
applied
during
impregnation
where
the
metal
contacts
with
the
solid
support
for
the
first
time.
However,
experiments
have
shown
that
drying
may
also
significantly
impact
the
metal
distribution
within
the
support.
Therefore,
to
achieve
a
desired
metal
profile
we
need
to
understand
both
impregnation
and
drying.
Controlling
the
drying
conditions
can
enhance
catalyst
performance.
PROJECT GOALS
The
goal
of
this
project
is
to
develop
a
fundamental
understanding
of
unit
operations
during
catalyst
preparation,
so
we
can
predict,
control
and
optimize
metal
distribution
and
dispersion
in
supported
catalysts.
Therefore,
we
can
provide
our
partners
with efficient tools to monitor and control the final quality of supported catalysts.
SUMMARY OF STUDIES
In
this
work
we
have
developed
a
theoretical
model
for
drying
which
we
have
validated
experimentally.
In
this
model,
we
have
taken
into
account
heat
transfer
from
the
hot
air
to
the
wet
support,
solvent
evaporation
in
the
support,
convective
flow
towards
the
support
external
surface
due
to
the
capillary
force,
as
well
as
metal
diffusion
and
deposition
due
to
adsorption
and
crystallization
(see
Figure
1a).
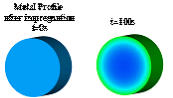
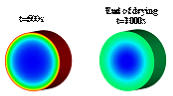
In
general,
the
convective
flow
is
the
main
driving
force
to
transport
the
metal
component
and
the
solvent
towards
the
supports
external
surface
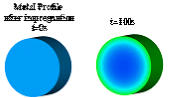
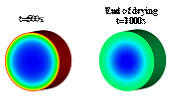
(t=500s
in
Figure
1b),
while
the
back-diffusion
causes
metal
to
transport towards the support center (t=1000s in Figure 1b).
Figure 1. (a) drying mechanism, (b) simulation of the evolution of the metal distribution during drying
We
also
developed
a
theoretical
model
to
predict
the
drying
process
for
high
metal
load
conditions;
this
was
accomplished
by
building
upon
a
model
that
was
established
for
low
metal
loadings.
It
is
found
that
the
drying
mechanisms
for
low
metal
loading
conditions
and
high
metal
loading
conditions
are
quite
different.
This
model
is
applicable
for
higher
concentrations
of
nickel
nitrate
(above
0.1
M).
It
included
the
effects
of
the
metal
concentration
on
the
solution
density,
viscosity,
surface
tension,
vapor
pressure
and
the
volume
ratio
of
metal.
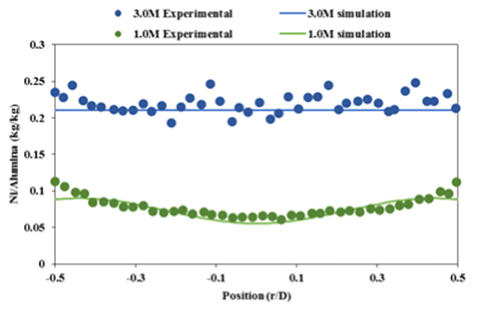
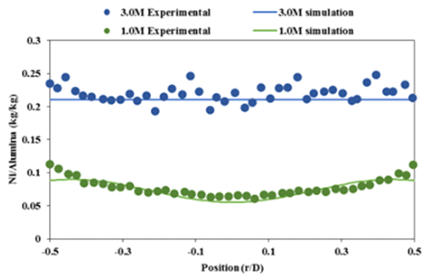
Good
agreement
was
found
between
experimental
and
simulation
post- drying metal distributions for this model using nickel nitrate. (see Figure 2).
Figure 2. Experimental results compared to post drying metal distributions using simulation for two different metal loadings
(1.0 M and 3.0 M). (T=80C, uniform initial condition)
In
our
work,
we
are
interested
in
investigating
the
importance
of
the
physical
properties
of
the
solid
support
(porosity,
pore
size
distribution,
particle
size)
and
liquid
solution
(pH,
ionic
strength,
initial
metal
precursor
concentration),
the
nature
of
interactions
that
exist
between
the
dissolved
metal
and
the
solid
support
(physical
adsorption,
crystallization,
ion
exchange,
film-breakage,
pore-blockage),
and
their
effects
on
the
distribution
and
dispersion
of
the
active
metal.
We
have
examined
the
distribution of various metals such as Nickel, Copper, Barium, and/or Palladium on Alumina (see Figure 3).
Figure 3. L - R: Porous alumina supports before impregnation, during impregnation and after impregnation followed by drying at 80C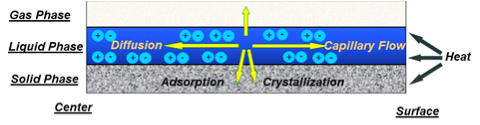

(a)
(b)
Last Updated: August 2023
PROJECT DESCRIPTION
BACKGROUNDSupported
catalysts
are
essential
components
in
a
variety
of
industrial
processes,
ranging
from
catalytic
converters
to
production
of
new
drugs.
The
performance
of
a
catalytic
process
is
intimately
related
to
the
catalyst
design
-
uniform,
egg-yolk,
egg
-
shell
and
egg
-
white
metal
profiles.
It
is
generally
believed
that
the
metal
profile
is
controlled
by
the
conditions
that
are
applied
during
impregnation
where
the
metal
contacts
with
the
solid
support
for
the
first
time.
However,
experiments
have
shown
that
drying
may
also
significantly
impact
the
metal
distribution
within
the
support.
Therefore,
to
achieve
a
desired
metal
profile
we
need
to
understand
both
impregnation
and
drying.
Controlling
the
drying
conditions
can enhance catalyst performance.
PROJECT GOALS
The
goal
of
this
project
is
to
develop
a
fundamental
understanding
of
unit
operations
during
catalyst
preparation,
so
we
can
predict,
control
and
optimize
metal
distribution
and
dispersion
in
supported
catalysts.
Therefore,
we
can
provide
our
partners
with
efficient
tools
to
monitor
and
control
the
final
quality
of
supported
catalysts.
SUMMARY OF STUDIES
In
this
work
we
have
developed
a
theoretical
model
for
drying
which
we
have
validated
experimentally.
In
this
model,
we
have
taken
into
account
heat
transfer
from
the
hot
air
to
the
wet
support,
solvent
evaporation
in
the
support,
convective
flow
towards
the
support
external
surface
due
to
the
capillary
force,
as
well
as
metal
diffusion
and
deposition
due
to
adsorption
and
crystallization
(see
Figure
1a).
In
general,
the
convective
flow
is
the
main
driving
force
to
transport
the
metal
component
and
the
solvent
towards
the
supports
external
surface
(t=500s
in
Figure
1b),
while
the
back-diffusion
causes
metal
to
transport
towards
the
support
center
(t=1000s in Figure 1b).
Figure 1. (a) drying mechanism, (b) simulation of the
evolution of the metal distribution during drying
We
also
developed
a
theoretical
model
to
predict
the
drying
process
for
high
metal
load
conditions;
this
was
accomplished
by
building
upon
a
model
that
was
established
for
low
metal
loadings.
It
is
found
that
the
drying
mechanisms
for
low
metal
loading
conditions
and
high
metal
loading
conditions
are
quite
different.
This
model
is
applicable
for
higher
concentrations
of
nickel
nitrate
(above
0.1
M).
It
included
the
effects
of
the
metal
concentration
on
the
solution
density,
viscosity,
surface
tension,
vapor
pressure
and
the
volume
ratio
of
metal.
Good
agreement
was
found
between
experimental
and
simulation
post-
drying
metal
distributions
for
this
model
using nickel nitrate. (see Figure 2).
Figure 2. Experimental results compared to post drying
metal distributions using simulation for two different metal
loadings (1.0 M and 3.0 M). (T=80C, uniform initial condition)
In
our
work,
we
are
interested
in
investigating
the
importance
of
the
physical
properties
of
the
solid
support
(porosity,
pore
size
distribution,
particle
size)
and
liquid
solution
(pH,
ionic
strength,
initial
metal
precursor
concentration),
the
nature
of
interactions
that
exist
between
the
dissolved
metal
and
the
solid
support
(physical
adsorption,
crystallization,
ion
exchange,
film-
breakage,
pore-blockage),
and
their
effects
on
the
distribution
and
dispersion
of
the
active
metal.
We
have
examined
the
distribution
of
various
metals
such
as
Nickel,
Copper,
Barium,
and/or
Palladium
on
Alumina
(see
Figure
3).
Figure 3. L - R: Porous alumina supports before impregnation, during impregnation and after impregnation followed by drying at 80C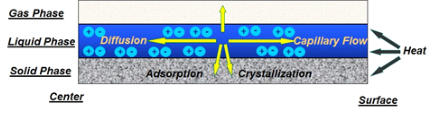

(a)
(b)














